The World Health Organisation (WHO) has endorsed the use of the long-acting injectable antiretroviral drug lenacapavir for HIV prevention. This twice-yearly injection is recommended as an additional option for pre-exposure prophylaxis (PrEP), offering a long-acting alternative to daily oral medications. It’s given once every six months, and according to experts, it offers sustained protection against the virus. NTV Kenya and Daily Nation reports
The announcement was made on Monday at the official opening of the 13th International AIDS Society Conference on HIV Science in Kigali, Rwanda, where the global organisation also unveiled new HIV treatment guidelines.

The endorsement of lenacapavir by WHO comes a few weeks after its approval by the US Food and Drug Administration (FDA).
“We have the tools and the knowledge to end Aids as a public health threat,” said Dr Meg Doherty, director of WHO’s Department of Global HIV, Hepatitis and STI Programmes.
“What we need now is bold implementation—rooted in equity and driven by communities.”
Echoing this optimism, WHO Director-General, Dr Tedros Adhanom Ghebreyesus, described lenacapavir as “the next best thing” to a HIV vaccine.
“With the FDA’s recent approval and WHO’s new guidance, we are taking a crucial step toward expanding access,” he said.
Welcoming the decision, Dr Patrick Amoth, director general for Health at the Ministry of Health, noted that the discreet, long-acting formulation of the new injectable PrEP could help address common challenges in HIV prevention—such as the burden of taking daily pills, frequent clinic visits, and the stigma linked to PrEP use.
“The challenge with daily PrEP is that missing a dose, even once or twice, can affect its effectiveness,” he said. “This new injection offers protection for six months with a single dose, making adherence much easier.”
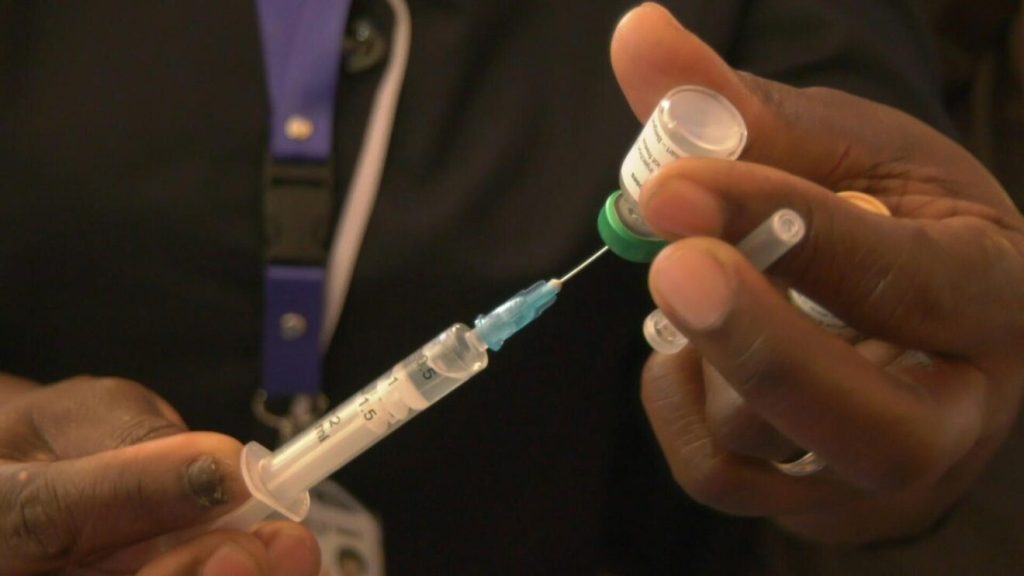
He added: “We’re proud that our country is among the first to adopt this breakthrough. The reason we’re aiming to roll it out in January is because we need time to update national guidelines, train healthcare workers, and set up the logistics around procurement, storage, and distribution. If those systems were already in place, we’d be ready to deploy it immediately.”
Long-acting HIV therapies and prevention tools are being hailed as a game-changer, particularly for vulnerable and underserved populations where daily medication can be difficult to maintain. Building on this momentum, the Medicines Patent Pool and ViiV Healthcare also announced an expanded licensing agreement that will open up access to long-acting cabotegravir, another injectable antiretroviral now recommended by WHO for HIV treatment.
“This is a significant step forward for equitable access,” said Esteban Burrone, director of Strategy, Policy and Market Access at the Medicines Patent Pool. “Communities have long asked for alternatives to daily pills. This proves that innovation and access can—and must—go hand in hand.”
Adding to the optimism, pharmaceutical giant MSD presented new Phase 2 trial data for MK-8527, a once-monthly oral pill for HIV prevention. The data, which shows a favourable safety and tolerability profile, supports the drug’s advancement into Phase 3 trials in Africa.
“Our upcoming EXPrESSIVE studies will evaluate MK-8527 across populations who would benefit most from PrEP,” said Dr Rebeca Plank, a distinguished scientist at MSD Research Laboratories. “Partnering with the Gates Foundation and the International Clinical Research Center, we are working to bring this promising option closer to communities.”
Despite these advancements, activists and global health advocates at Kigali conference were quick to warn that science alone will not solve the HIV epidemic.
“Long-acting HIV prevention and treatment can only transform lives if people can actually access them,” said Yvette Raphael, executive director of Advocates for the Prevention of HIV and Aids. “We’ve seen it before—new medicines arrive, but the people who need them most are left behind. We need concrete plans, real funding, and community trust to close that gap.”
This message was reinforced by Mary Mahy, UNAIDS director for Data Impact, who presented data showing that by the end of 2024, 73 per cent of people living with HIV had achieved viral suppression—a key milestone in both individual health and reducing transmission.
However, Mahy warned that progress is increasingly threatened by a global funding crisis.
UNAIDS Executive Director Winnie Byanyima emphasised the urgency of the situation in her opening address. “We are facing a massive disruption in international HIV financing,” she said. “This has created systemic shocks that are jeopardising HIV treatment and prevention programmes globally.”
Dr Beatriz Grinsztejn, president of the International AIDS Society, issued a call to action, urging global leaders, funders, and institutions to invest in turning these scientific breakthroughs into accessible, real-world solutions for everyone.
“We are at a pivotal moment,” she said. “New WHO guidelines, licensing agreements, and research innovations show what’s possible. But science only matters when it reaches people. Now is the time to invest—urgently and equitably—so these life-changing options become part of routine HIV care everywhere.”
The conference, which ends on Thursday, is hosting hundreds of sessions and presentations aimed at translating latest scientific advances into real-world impact. With a strong focus on regions and populations most affected by HIV, the conference brings together over 4,000 participants from around the world—both in person and online—to share research, policy insights, and practical solutions.



